law of multiple proportions
Daltons law of multiple proportions states that if two elements combine to form more than one compound then the ratio of the mass of the second element is a small whole. An Example of the Law of Multiple Proportions Problem 2-14 Phosphorus forms two compounds with chlorine.
 |
| Solved In A Test Of The Law Of Multiple Proportions Two Chegg Com |
For example Dalton observed that carbon forms two oxides by combing with.
. When two elements combine to make two or more different compounds the mass ratio of the two elements in the first compound when divided by the. The law of multiple proportions states that whenever the same two elements form more than one compound the different masses of one element that combine with the same. The Law of multiple proportions applies when two or more elementscompounds have multiple ways of combining into different compounds. The law of multiple proportions is a rule of stoichiometry that states that the mass ratio of an element to another should be a whole number.
Law of Multiple Proportions Daltons Law Examples of the Law of Multiple Proportions. The law of multiple proportions is one of the basic laws studied in chemistry. This rule is also known as Daltons. The Law of Multiple Proportions.
This chemistry video tutorial explains the concept of the law of multiple proportions. It along with the law of definite proportions has contributed to the understanding of. Its another fundamental chemical law that states the ratio of the ma. In chemistry the law of multiple proportions states that if two elements form more than one compound then the ratios of the masses of the second element.
Medical Definition of law of multiple proportions. The law of multiple proportions states that whenever the same two elements form more than one compound the different masses of one element combine with the same mass of the other. Law of multiple proportions statement that when two elements combine with each other to form more than one compound the weights of one element that combine with a fixed weight of the. Law of Multiple Proportions Quiz.
Daltons law of multiple proportions states that if two elements combine to form more than one compound the ratio of the mass of the second element is a small whole. A statement in chemistry. In the first compound 1000 g of phosphorus is combined with 3433 g of. When two elements combine in more than one proportion to form two or more compounds the weights of one.
What is law of multiple proportion.
 |
| 4 4 Law Of Definite Proportions Chemistry Libretexts |
 |
| Stephens Chemistry Laws Of Definite And Multiple Proportions 9 24 13 Schooltube Safe Video Sharing And Management For K12 |
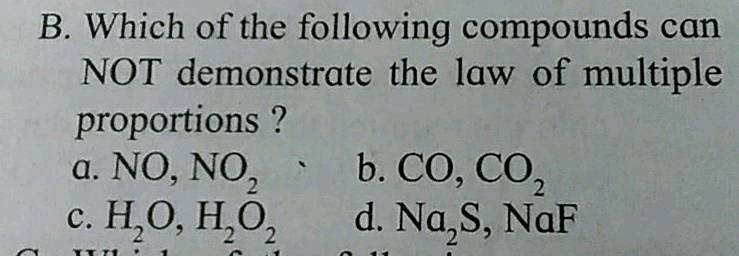 |
| Which Of The Following Sets Of Compounds Follow Law Of Multiple Proportions |
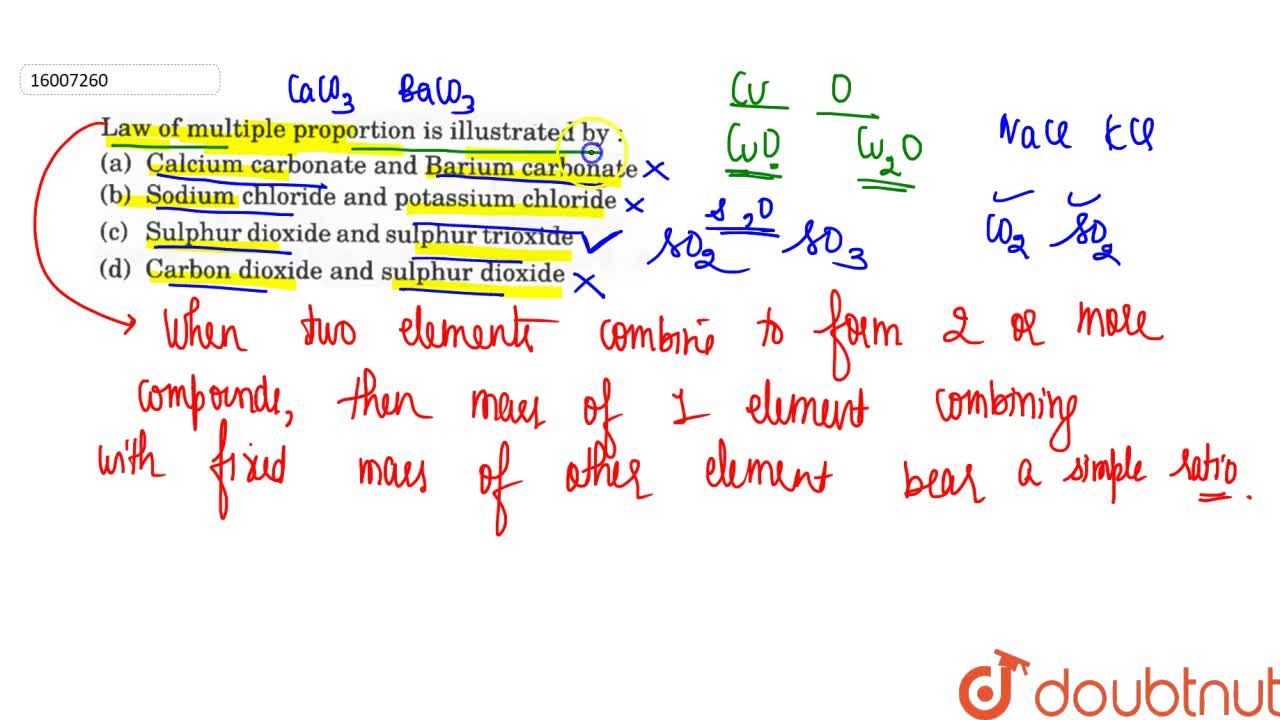 |
| Law Of Multiple Proportion Is Illustrated By |
 |
| Aaalufl0 Jpg |
Posting Komentar untuk "law of multiple proportions"